Abstract
Background: Extended T-cell culture periods in vitro deplete the CAR-T final product of naive and stem cell memory T-cell (T scm) subpopulations that are associated with improved antitumor efficacy. YTB323 is an autologous CD19-directed CAR-T cell therapy with dramatically simplified manufacturing, which eliminates complexities such as long culture periods. This improved T-Charge™ process preserves T-cell stemness, an important characteristic closely tied to therapeutic potential, which leads to enhanced expansion ability and greater antitumor activity of CAR-T cells.
Methods: The new T-Charge TM manufacturing platform, which reduces ex vivo culture time to about 24 hours and takes <2 days to manufacture the final product, was evaluated in a preclinical setting. T cells were enriched from healthy donor leukapheresis, followed by activation and transduction with a lentiviral vector encoding for the same CAR used for tisagenlecleucel. After ≈24 hours of culture, cells were harvested, washed, and formulated (YTB323). In parallel, CAR-T cells (CTL*019) were generated using a traditional ex vivo expansion CAR-T manufacturing protocol (TM process) from the same healthy donor T cells and identical lentiviral vector. Post manufacturing, CAR-T products were assessed in T-cell functional assays in vitro and in vivo, in immunodeficient NSG mice (NOD-scid IL2Rg-null) inoculated with a pre-B-ALL cell line (NALM6) or a DLBCL cell line (TMD-8) to evaluate antitumor activity and CAR-T expansion. Initial data from the dose escalation portion of the Phase 1 study will be reported separately.
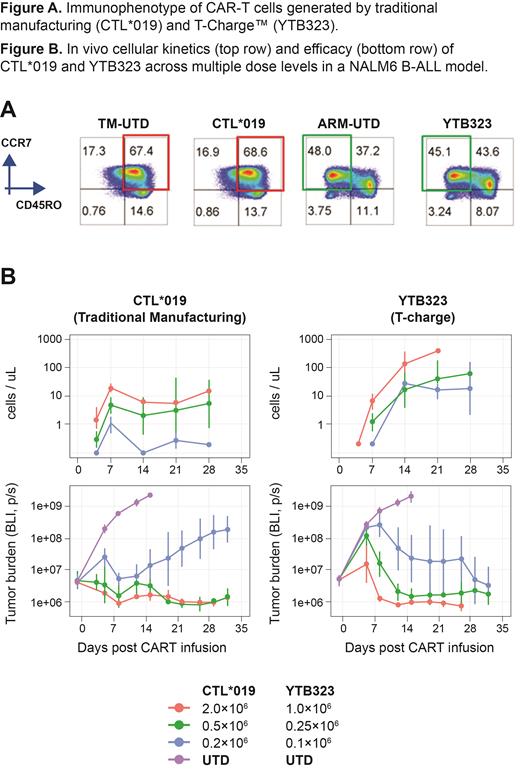
Results: YTB323 CAR-T products, generated via this novel expansionless manufacturing process, retained the immunophenotype of the input leukapheresis; specifically, naive/T scm cells (CD45RO -/CCR7 +) were retained as shown by flow cytometry. In contrast, the TM process with ex vivo expansion generated a final product consisting mainly of central memory T cells (T cm) (CD45RO +/CCR7 +) (Fig A). Further evidence to support the preservation of the initial phenotype is illustrated by bulk and single-cell RNA sequencing experiments, comparing leukapheresis and final products from CAR-Ts generated using the T-Charge™ and TM protocols. YTB323 CAR-T cell potency was assessed in vitro using a cytokine secretion assay and a tumor repeat stimulation assay, designed to test the persistence and exhaustion of the cell product. YTB323 T cells exhibited 10- to 17-fold higher levels of IL-2 and IFN-γ secretion upon CD19-specific activation compared with CTL*019. Moreover, YTB323 cells were able to control the tumor at a 30-fold lower Effector:Tumor cell ratio and for a minimum of 7 more stimulations in the repeat stimulation assay. Both assays clearly demonstrated enhanced potency of the YTB323 CAR-T cells in vitro. The ultimate preclinical assessment of the YTB323 cell potency was through comparison with CTL*019 regarding in vivo expansion and antitumor efficacy against B-cell tumors in immunodeficient NSG mouse models at multiple doses. Expansion of CD3+/CAR+ T-cells in blood was analyzed weekly by flow cytometry for up to 4 weeks postinfusion. Dose-dependent expansion (C max and AUC 0-21d) was observed for both YTB323 and CTL*019. C max was ≈40-times higher and AUC 0-21d was ≈33-times higher for YTB323 compared with CTL*019 across multiple doses. Delayed peak expansion (T max) of YTB323 by at least 1 week compared with CTL*019 was observed, supporting that increased expansion was driven by the less differentiated T-cell phenotype of YTB323. YTB323 controlled NALM6 B-ALL tumor growth at a lower dose of 0.1×10 6 CAR+ cells compared to 0.5×10 6 CAR+ cells required for CTL*019 (Fig B). In the DLBCL model TMD-8, only YTB323 was able to control the tumors while CTL*019 led to tumor progression at the respective dose groups. This ability of YTB323 cells to control the tumor at lower doses confirms their robustness and potency.
Conclusions: The novel manufacturing platform T-Charge™ used for YTB323 is simplified, shortened, and expansionless. It thereby preserves T-cell stemness, associated with improved in vivo CAR-T expansion and antitumor efficacy. Compared to approved CAR-T therapies, YTB323 has the potential to achieve higher clinical efficacy at its respective lower doses. T-Charge™ is aiming to substantially revolutionize CAR-T manufacturing, with concomitant higher likelihood of long-term deep responses.
Engels: Novartis: Current Employment, Current equity holder in publicly-traded company. Zhu: Novartis: Current Employment, Current equity holder in publicly-traded company. Yang: Novartis: Current Employment, Patents & Royalties. Price: Novartis: Current Employment. Sohoni: Novartis: Current Employment. Stein: Novartis: Current Employment. Parent: Novartis: Ended employment in the past 24 months; iVexSol, Inc: Current Employment. Greene: iVexSol, Inc: Current Employment, Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Niederst: Novartis: Current Employment, Current equity holder in publicly-traded company. Whalen: Novartis: Current Employment. Orlando: Novartis: Current Employment. Treanor: Novartis: Current Employment, Current holder of individual stocks in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties: no royalties as company-held patents. Brogdon: Novartis Institutes for Biomedical Research: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal